-40%
2g Thidiazuron TDZ 98%. Stop Overpaying. Higher/Better Quality Than Caissonlab
$ 6.1
- Description
- Size Guide
Description
2g Thidiazuron TDZ 98%. Production Date12/6/20 Expiration Date 12/6/22Stop buying at expensive labs that absolutely overcharge you. My TDZ is Higher/Better quality than Caissonlabs.com, they only offer 95% and they charge 1 for 1g. Here's what they sell 👇 I copied from their website. If you don't have the confidence that my TDZ is better or same quality than try buying 2g and see for yourself. It'll cost you pennies and save you a lot of money in near future.
"
Thidiazuron - Powder, 1 GM
9.01
>95%; CAS Number: 51707-55-2; Formula: C9H8N4OS; Formula Weight: 220.25. This item is considered a Dangerous Good for shipping purposes and cannot be shipped to a residential address. A Dangerous Good Fee may be added per box to the shipping cost of this item".
____________________________________________________
Thidiazuron is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide (DMF), Solubilize at 1 mg/mL in 0.1N KOH
Thidiazuron is a urea-type highly active cytokinin, which can strongly promote cell division at low concentrations, promote photosynthesis, and accelerate the efficient transport of photosynthetic products to fruits. It can be used to promote fruit setting and fruit expansion, to keep leaves green, to delay leaf senescence, and to induce callus differentiation and budding. It is a cotton decidant at high concentration. After being absorbed by the cotton plant leaves, it can promptly promote the natural formation of the detached tissue between the petiole and the stem to leave the leaf. Improve cotton rank.
Uses & Benefits:
Regulate growth and increase yield - Spraying 3mg / L Thidiazuron solution once during the division and flowering stages of rice crops can improve the quality of rice agronomic traits, increase the number of panicles and seed setting of rice, reduce the number of grains, and increase yields up to 16%
Regulate growth and increase yield - Spray grape plants with 4-6mg / L Thidiazuron solution about 5 days after the grapes bloom, and use it a second time every 10 days to promote fruit setting and fruit expansion and increase yield.
Regulate growth and increase yield - In the center of the apple tree blossom 10% - 20% and full bloom period, each application with 2-4mg / L Thidiazuron solution can promote fruit setting.
Defoliant - When more than 60% of cotton peach is cracked, spray the leaves evenly according to 15-30mg / m2 Thidiazuron and water to promote defoliation.
Thidizuron (TDZ) is among the most active cytokinin-like substances for woody plant tissue culture. It facilitates efficient micropropagation of many recalcitrant woody species. Low concentrations (<1 µM) can induce greater axillary proliferation than many other cytokinins; however, TDZ may inhibit shoot elongation. In some cases it is necessary to transfer shoots to an elongation medium containing a lower level of TDZ and/or a less active cytokinin. At concentrations higher than 1 µM, TDZ can stimulate the formation of callus, adventitious shoots or somatic embryos. Subsequent rooting of microshoots may be unaffected or slightly inhibited by prior exposure to TDZ. The main undesirable side effect of TDZ is that cultures of some species occasionally form fasciated shoots. The high cytokinin activity and positive response of woody species to TDZ have established it as among the most active cytokinins forin vitro manipulation of many woody species.
__________________________________________________
A MICROPROPAGATION SYSTEM FOR CLONING OF HEMP, CANNABIS, FOR BOTH MEDICAL AND INDUSTRIAL USE WITH THE HELP OF TDZ THIDIAZURON.
Abstract
&
Figures
Induction of high-frequency shoot regeneration using nodal segments containing axillary buds from a 1-yr-old mother plants of Cannabis sativa was achieved on Murashige and Skoog (MS) medium containing 0.05–5.0μM thidiazuron. The quality and quantity of regenerants were better with thidiazuron (0.5μM thidiazuron) than with benzyladenine or kinetin. Adding 7.0μM of gibberellic acid into a medium containing 0.5μM thidiazuron slightly increased shoot growth. Elongated shoots when transferred to half-strength MS medium supplemented with 500mg l−1 activated charcoal and 2.5μM indole-3-butyric acid resulted in 95% rooting. The rooted plants were successfully acclimatized in soil. Following acclimatization, growth performance of 4-mo-old in vitro propagated plants was compared with ex vitro vegetatively grown plants of the same age. The photosynthesis and transpiration characteristics were studied under different light levels (0, 500, 1,000, 1,500, or 2,000μmol m−2 s−1). An increase in photosynthesis was observed with increase in the light intensity up to 1,500μmol m−2 s−1 and then decreased subsequently at higher light levels in both types of plants. However, the increase was more pronounced at lower light intensities below 500μmol m−2 s−1. Stomatal conductance and transpiration increased with light intensity up to highest level (2000μmol m−2 s−1) tested. Intercellular CO2 concentration (C i) and the ratio of intercellular CO2 concentration to ambient CO2 (C i/C a) decreased with the increase in light intensity in both in vitro as well as ex vitro raised plants. The results show that in vitro propagated and hardened plants were functionally comparable to ex vitro plants of same age in terms of gas and water vapor exchange characteristics, within the limits of this study.
__________________________________________________
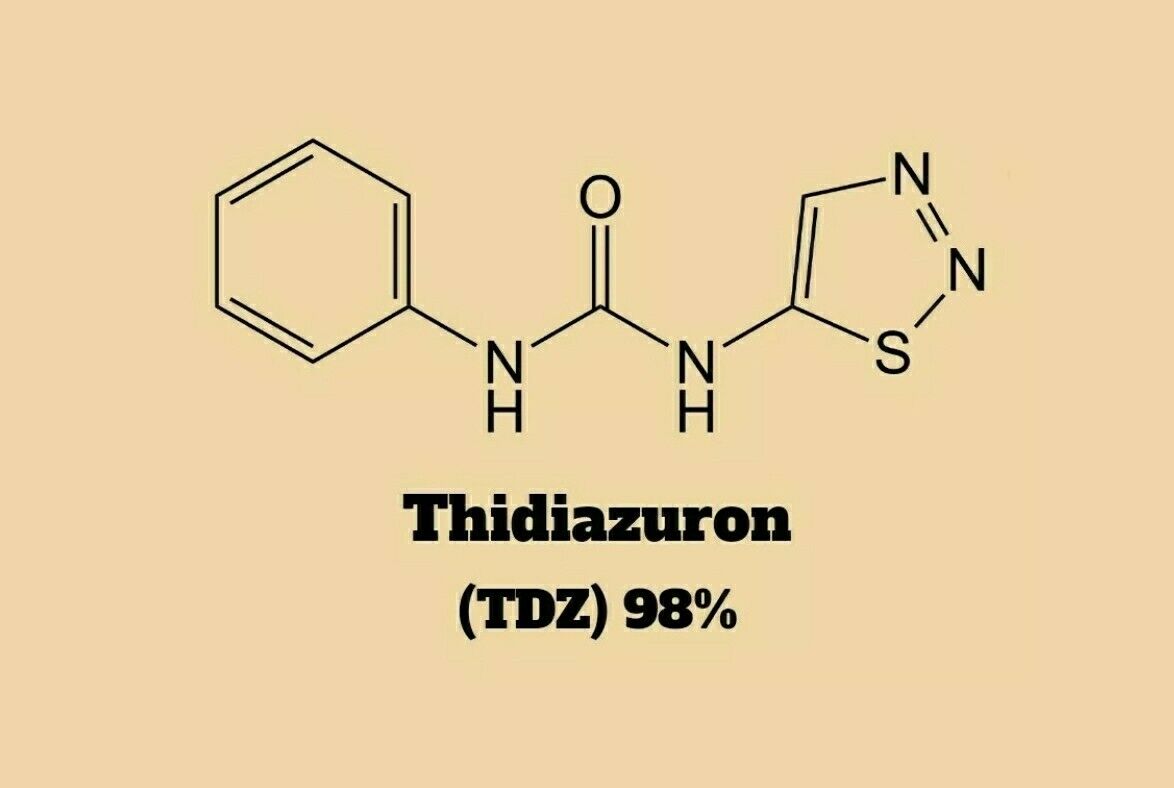
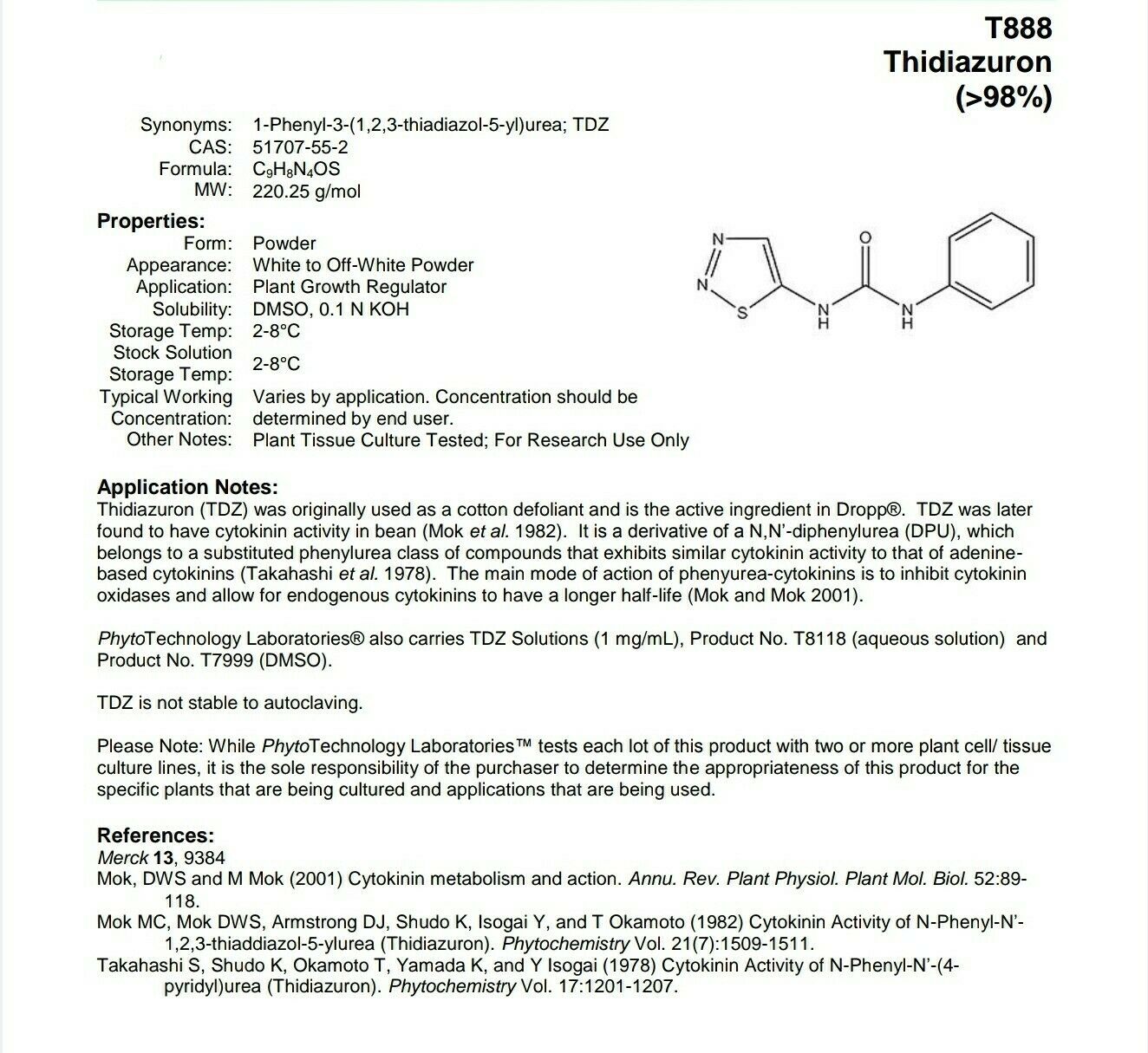
Synonyms: 1-Phenyl-3-(1,2,3-thiadiazol-5-yl)urea; TDZ
CAS: 51707-55-2
Formula: C9H8N4OS
MW: 220.25 g/mol
Properties:
Form: Powder
Appearance: White to Off-White Powder
Application: Plant Growth Regulator
Solubility: DMSO, 0.1 N KOH
Storage Temp: 2-8°C
Stock Solution
Storage Temp: 2-8°C
Typical Working
Concentration:
Varies by application. Concentration should be
determined by end user.
Other Notes: Plant Tissue Culture Tested; For Research Use Only
Application Notes:
Thidiazuron (TDZ) was originally used as a cotton defoliant and is the active ingredient in Dropp®. TDZ was later
found to have cytokinin activity in bean (Mok et al. 1982). It is a derivative of a N,N’-diphenylurea (DPU), which
belongs to a substituted phenylurea class of compounds that exhibits similar cytokinin activity to that of adenine-
based cytokinins (Takahashi et al. 1978). The main mode of action of phenyurea-cytokinins is to inhibit cytokinin
oxidases and allow for endogenous cytokinins to have a longer half-life (Mok and Mok 2001).
PhytoTechnology Laboratories® also carries TDZ Solutions (1 mg/mL), Product No. T8118 (aqueous solution) and
Product No. T7999 (DMSO).
TDZ is not stable to autoclaving.
Please Note: While PhytoTechnology Laboratories™ tests each lot of this product with two or more plant cell/ tissue
culture lines, it is the sole responsibility of the purchaser to determine the appropriateness of this product for the
specific plants that are being cultured and applications that are being used.
References:
Merck 13, 9384
Mok, DWS and M Mok (2001) Cytokinin metabolism and action. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52:89-
118.
Mok MC, Mok DWS, Armstrong DJ, Shudo K, Isogai Y, and T Okamoto (1982) Cytokinin Activity of N-Phenyl-N’-
1,2,3-thiaddiazol-5-ylurea (Thidiazuron). Phytochemistry Vol. 21(7):1509-1511.
Takahashi S, Shudo K, Okamoto T, Yamada K, and Y Isogai (1978) Cytokinin Activity of N-Phenyl-N’-(4-
pyridyl)urea (Thidiazuron). Phytochemistry Vol. 17:1201-1207.
__________________________________________________
Thidiazuron (TDZ) increases fruit set and yield of ‘Hosui’and ‘Packham’s Triumph’pear trees
Mateus S Pasa, CARINA P SILVA, Bruno Carra, Alberto F Brighenti, ANDRÉ LUIZ K SOUZA, Jose Luiz Petri
Anais da Academia Brasileira de Ciências 89 (4), 3103-3110, 2017
The low fruit set is one of the main factors leading to poor yield of pear orchards in Brazil. The exogenous application of thidiazuron (TDZ) and aminoethoxyvinilglycine (AVG) has shown promising results in some pear cultivars and other temperate fruit trees. The objective of this study was to evaluate the effect of TDZ and AVG on fruit set, yield, and fruit quality of ‘Hosui’ and ‘Packham’s Triumph’ pears. The study was performed in a commercial orchard located in São Joaquim, SC. Plant material consisted of ‘Hosui’ and ‘Packham’s Triumph’ pear trees grafted on Pyrus calleryana. Treatments consisted on different rates of TDZ (0 mg L-1, 20 mg L-1, 40 mg L-1 and 60 mg L-1) sprayed at full bloom for both cultivars. An additional treatment of AVG 60 mg L-1 was sprayed one week after full bloom in ‘Hosui’. The fruit set, number of fruit per tree, yield, fruit weight, seed number, and fruit quality attributes were assessed. Fruit set and yield of both cultivars are consistently increased by TDZ, within the rates of 20 to 60 mg L-1. Besides, its application increased fruit size of ‘Hosui’ and did not negatively affect fruit quality attributes of both cultivars.
Abstract
Cannabis sativa is usually clonally propagated from plants in the vegetative phase. However, phenotypic traits such as yield and chemical composition can only be assessed in unfertilized plants reaching the end of their life cycle and there are no peer-reviewed methods to propagate flowering plants. In this study, immature (three cultivars) and mature (one cultivar) floral explants were cultured on thidiazuron and shoot development was observed in both the immature and mature explants. This provides the first report of micropropagation from floral tissues in C. sativa and will enable plants to be clonally propagated up to the date of harvest.
Introduction
Cannabis sativa L. has recently gained increased acceptance for medicinal and recreational use in many countries. While phenotypically diverse, medicinal and recreational cultivars are generally determinate, dioecious, outcrossing, annual plants. Plants grown from seed demonstrate high degrees of variability in respect to chemical composition, growth habit, and agronomic traits (de Meijer et al. 2003; Caplan et al. 2017), so clonal propagation is commonly used to maintain uniformity.
Traditionally, this is done using stem cuttings, but more recent reports describe methods for in vitro propagation through meristem proliferation and de novo regeneration (Reviewed in Lata et al. 2017). To produce cuttings, mother plants are maintained in a vegetative state under long-day conditions to provide vegetative shoots. While there are some reports of propagation from flowering plants in the grey literature, referred to as “monster cropping”, there are no known peer-reviewed reports of propagation from flowering plants.
Early studies attempted to regenerate Cannabis plants from stem, leaf, and petiole explants with limited success (Reviewed in Lata et al. 2017). Low levels of shoot regeneration were obtained from cotyledon, epicotyl, or stem explants and higher levels have been reported from leaf explants. Perhaps the most practical system available for micropropagation uses meristem tips or axillary buds to multiply genetically consistent plants. To date, all reports of in vitro plant regeneration have been from vegetative plant material and may not be applicable to plants in the reproductive phase.
While propagation from vegetative tissues is suitable to multiply plants with known characteristics, many important traits (chemical profile, organoleptic properties, etc.) are only expressed in unfertilized (seedless) plants during the late reproductive phase. This has important implications for selecting new germplasm and establishing large-scale breeding programs, as there are no reliable methods to propagate them after they have reached full maturity. To circumvent this issue, cuttings can be taken from each plant before inducing flowering and be maintained in a vegetative state while the original seedlings are grown to maturity and selected for floral traits. However, this approach is inefficient, as it requires each genotype to be maintained in two locations during the selection process, adding significant costs in labour, production space, and operating expenses.
The lack of methods to propagate plants in the reproductive phase also presents issues in the development and large-scale clonal propagation of day-neutral cultivars. These cultivars are not photoperiod sensitive and enter the reproductive phase based on their physiological maturity regardless of day length. While they offer several agronomic advantages, they cannot be maintained as mother plants and are difficult to clonally propagate. To facilitate more efficient plant breeding and clonal propagation of day-neutral cultivars, a vegetative propagation system using floral tissues would be ideal. The objective of the current study was to evaluate the potential of floral explants for micropropagation of C. sativa.